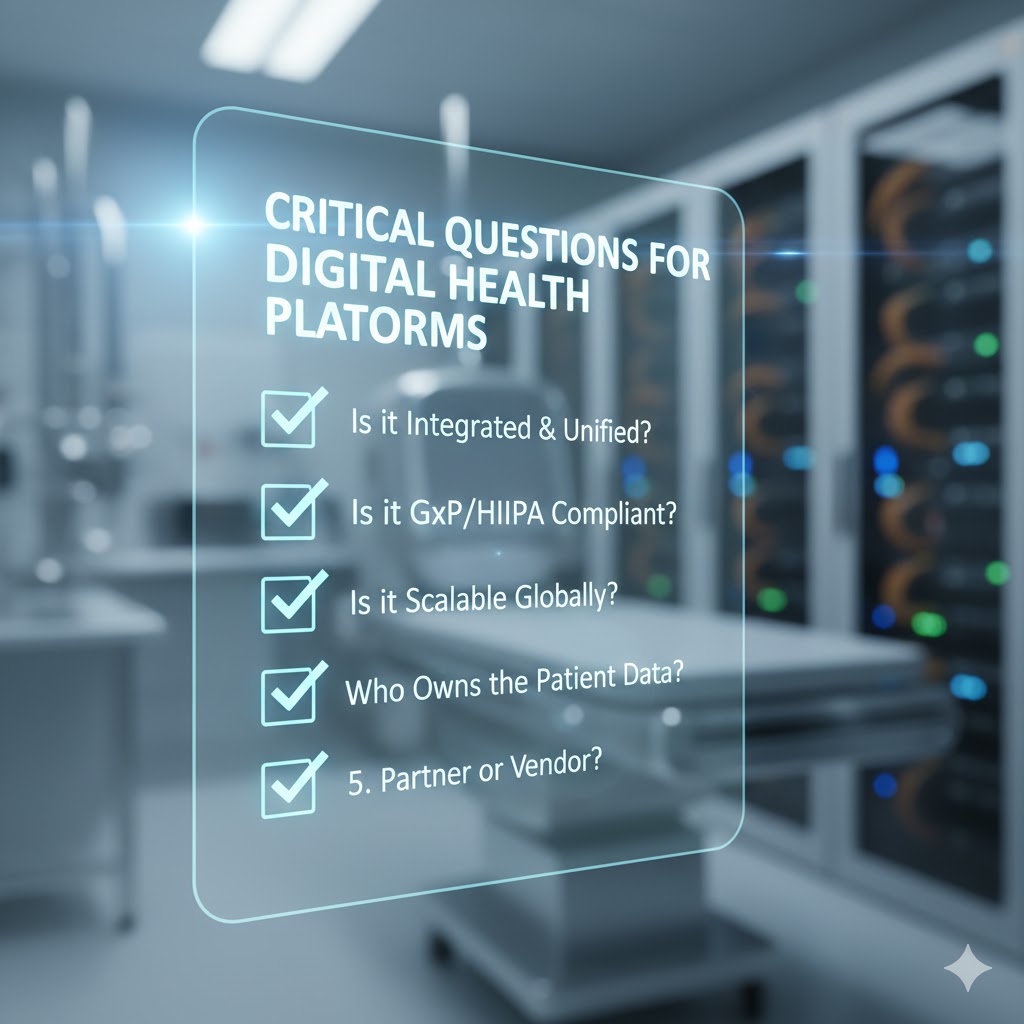
28 Top Advarra Competitors & Alternatives
Clinical trials are critical to the development of new medical treatments, procedures, and therapies. They provide the evidence that regulators and healthcare providers require to determine the safety and efficacy of new interventions. However, conducting clinical trials is a complex and resource-intensive process that requires careful planning, execution, and management.
Clinical trial management refers to the process of planning, organizing, and coordinating all aspects of a clinical trial. This includes identifying eligible participants, designing the study protocol, ensuring ethical and regulatory compliance, recruiting and training investigators, collecting and analyzing data, and reporting the results.
Effective clinical trial management is essential to ensure that trials are conducted efficiently, safely, and ethically, and that the results are reliable and can be used to inform clinical practice. It involves the collaboration of multiple stakeholders, including sponsors, investigators, regulators, ethics committees, and participants.
In recent years, the field of clinical trial management has undergone significant changes, driven by advances in technology, changes in regulations, and increasing public awareness of the importance of clinical research. As a result, there is a growing demand for skilled professionals who can manage clinical trials effectively and efficiently.
That said, here are a few top players in the field of clinical trial management that you must consider for your next clinical trial.
1. Mahalo Health vs. Advarra

When it comes to clinical trial management, there are many options available to researchers, including Mahalo Health and Advarra. Mahalo Health is an electronic platform designed to streamline clinical trial management and provide real-time access to data. The platform is highly intuitive and user-friendly, making it easy for researchers to design and build their own studies. One of the most significant advantages of Mahalo Health is its flexibility, which allows it to be used for a wide range of clinical trials.
Advarra, on the other hand, is a comprehensive clinical research organization (CRO) that provides end-to-end services for clinical trials. This includes everything from study design to data management, patient recruitment, and regulatory compliance. Advarra is known for its rigorous quality control measures and its ability to deliver high-quality data in a timely and efficient manner. One of the most significant advantages of Advarra is its extensive experience and expertise in the clinical trial industry.
When comparing Mahalo Health vs Advarra, it's essential to consider the specific needs of the researcher and the trial they are conducting. Mahalo Health is a great option for researchers who need a flexible, user-friendly platform for managing their clinical trials. However, for larger, more complex trials, Advarra's end-to-end services and extensive expertise may be a better fit.
Another important factor to consider is pricing. Mahalo Health offers subscription-based pricing, while Advarra's pricing varies depending on the specific services required. In terms of customer support, Mahalo Health has a solid reputation for providing excellent support to its clients.
Ultimately, choosing between Mahalo Health vs Advarra will depend on the specific needs of the researcher and the trial they are conducting. Both platforms have their strengths and weaknesses, so it's important to carefully consider all options before making a decision.
However, with its user-friendly interface and flexibility, Mahalo Health is a strong contender in the clinical trial management space.
2. Medable vs Advarra
Advarra is a well-known name in the clinical trial management industry, and it's no surprise that they have competitors in the market. One such competitor is Medable. Medable is a cloud-based platform that offers a range of eClinical tools for clinical research, including modules for patient engagement, remote monitoring, and decentralized trials. While Advarra is a robust and reliable platform, Medable has the advantage of being more comprehensive, making it an attractive option for researchers who need a full suite of tools for their clinical trials.
However, one advantage that Advarra has over Medable is its extensive experience in the industry. Advarra has been around for over 20 years, and during that time, they have built a reputation for providing excellent support and service to their clients. This experience and expertise make Advarra a trusted partner for researchers and clinical trial sites.
Another factor that sets Advarra apart from its competitors is its commitment to compliance and ethical research. Advarra's team of experts stays up to date on all the latest regulations and guidelines to ensure that their clients are conducting their clinical trials ethically and responsibly. Advarra's focus on compliance and ethics makes it an attractive choice for researchers who value these principles.
In conclusion, while Advarra has competitors like Medable in the market, it remains a robust and reliable platform with a reputation for excellent service and expertise in the industry. While Medable may be more comprehensive, Advarra's extensive experience and commitment to compliance and ethics make it an attractive choice for researchers and clinical trial sites. Ultimately, the choice between Advarra and its competitors will depend on the specific needs and requirements of the individual clinical trial.
3. Veeva Vault vs. Advarra
When it comes to managing clinical trials, two popular tools that come to mind are Veeva Vault and Advarra. Veeva Vault is an enterprise content management platform that offers a range of features, including electronic trial master file (eTMF) management, clinical data management, and quality management. Veeva Vault is known for its intuitive user interface, customizable workflows, and real-time collaboration features.
On the other hand, Advarra is a comprehensive research compliance and quality management platform that offers services and solutions for every stage of the clinical research process. Advarra provides a range of features, including study startup, regulatory compliance, data and safety monitoring, and quality management. Advarra is known for its highly configurable platform, comprehensive training and support, and expertise in research compliance.
Comparing Veeva Vault vs. Advarra, both platforms offer valuable features to help researchers streamline their workflows and manage clinical trials more efficiently. However, Veeva Vault is better suited for researchers who need a platform specifically designed for clinical trial management. At the same time, Advarra is better suited for researchers who need a comprehensive platform that can handle compliance and quality management across multiple studies.
In terms of pricing, both Veeva Vault and Advarra offer custom pricing based on the specific needs of the client. Veeva Vault pricing is typically based on the number of users, while Advarra pricing is based on the specific services and solutions needed. Both platforms offer enterprise-level pricing for large organizations and have a reputation for providing excellent customer support and training to their clients. Ultimately, choosing between Veeva Vault vs Advarra will depend on the specific needs of the research organization and the trials they are conducting.
4. REDCap vs. Advarra
When it comes to running clinical trials, two popular options for researchers are RedCap and Advarra. RedCap is a web-based platform developed by Vanderbilt University that allows researchers to manage their study data in a secure and customizable environment. One of the most significant advantages of RedCap is its flexibility, as it can be used for a wide range of clinical trials. Additionally, it offers advanced features such as electronic data capture, real-time data monitoring, and the ability to integrate with other clinical trial tools.
In contrast, Advarra is a comprehensive clinical research organization that offers a wide range of services, including regulatory consulting, IRB review, and technology solutions. Advarra's technology platform is designed to streamline the clinical trial process, from study startup to closeout, and includes features such as data management, safety monitoring, and electronic data capture. One of the most significant advantages of Advarra is its comprehensive suite of services, which allows researchers to manage all aspects of their clinical trial in one place.
When comparing RedCap vs Advarra, it's important to consider the specific needs of your clinical trial. RedCap is an excellent option for researchers who want a customizable platform that can be tailored to their specific study requirements. It also offers a range of advanced features that can help researchers manage their data more efficiently. On the other hand,
Advarra is a better choice for researchers who need a comprehensive suite of services, including regulatory consulting and IRB review. It's also a good option for researchers who want to manage all aspects of their clinical trial in one place.
In terms of pricing, RedCap is a free, open-source platform that can be downloaded and installed on a local server or hosted on the cloud. Advarra, on the other hand, offers custom pricing based on the specific needs of each client. While RedCap may be more cost-effective for smaller studies, Advarra's comprehensive suite of services may provide better value for larger and more complex trials.
In conclusion, when choosing between RedCap vs Advarra, researchers must carefully consider their specific needs and the requirements of their clinical trial. Both platforms offer valuable features and can be an asset to clinical trial management, but the choice ultimately depends on the individual researcher's needs.
5. BioClinica ICL vs. Advarra
When it comes to running clinical trials, BioClinica and Advarra are two popular options that offer a range of features and benefits. BioClinica is a cloud-based platform that provides end-to-end solutions for clinical trials, including data management, imaging, and eClinical technologies. One of the significant advantages of BioClinica is its expertise in medical imaging, making it a popular choice for researchers working on trials that require complex imaging data.
On the other hand, Advarra is a comprehensive clinical research organization (CRO) that provides a full range of services to support clinical trials, including regulatory consulting, site selection, and patient recruitment. Advarra's extensive network of research sites and experience in managing complex trials make it a popular choice for researchers who need additional support and expertise beyond just technology.
When comparing BioClinica vs Advarra, it's essential to consider the specific needs of your trial. BioClinica is an excellent option for researchers who need a robust imaging platform and eClinical tools to manage their data. In contrast, Advarra is a better choice for researchers who need a full-service CRO with expertise in regulatory compliance, site selection, and patient recruitment.
It's important to note that both BioClinica and Advarra offer customized pricing based on the specific needs of your trial. BioClinica's pricing is based on the number of imaging studies and the number of patients enrolled in the trial, while Advarra's pricing is based on the scope of services required. In terms of customer support, both platforms offer dedicated support teams and excellent customer service.
In conclusion, the choice between BioClinica vs Advarra ultimately depends on the needs of your specific trial. If you require specialized imaging capabilities and eClinical tools, BioClinica is an excellent option. On the other hand, if you need a full-service CRO with expertise in regulatory compliance and patient recruitment, Advarra is the way to go. Both platforms offer valuable features and benefits to support clinical trial management, and the right choice will depend on your unique needs and requirements.
6. Clinion vs. Advarra
Clinion and Advarra are two of the most popular software solutions for managing clinical trials. Clinion offers a suite of tools for randomization and trial supply management, as well as electronic data capture (EDC) and patient engagement. Advarra, on the other hand, provides a range of services for institutional review boards (IRBs) and research compliance.
When comparing Clinion vs Advarra, it's important to consider the specific needs of your clinical trial. Clinion is a great option for researchers who require advanced tools for randomization and trial supply management, as well as EDC and patient engagement. The platform is highly configurable, allowing users to tailor it to the unique requirements of their study. One of the significant advantages of Clinion is its ability to integrate with other clinical trial management systems, such as EDC and electronic patient-reported outcomes (ePRO) systems.
In contrast, Advarra is a better option for researchers who require services related to IRBs and research compliance. Advarra offers a comprehensive suite of services, including protocol development, site feasibility assessments, and study start-up support. The platform also provides ongoing support for IRB submissions, protocol amendments, and adverse event reporting. One of the most significant advantages of Advarra is its experience and expertise in the field of research compliance, making it an excellent choice for complex studies involving multiple sites and sponsors.
Ultimately, when choosing between Clinion vs Advarra, it's important to consider your specific needs and the requirements of your clinical trial. Both platforms have their strengths, and choosing the right one will depend on factors such as the size and complexity of your study, your budget, and your level of experience with clinical trial management systems. Overall, both Clinion and Advarra are powerful and reliable solutions that can help researchers streamline their workflows and run more efficient clinical trials.
7. Clinplus CTMS vs. Advarra
Advarra and Clinplus are two leading clinical trial management systems, each with its own unique strengths and weaknesses.
Advarra is a comprehensive platform designed to help researchers streamline the entire clinical trial process, from study startup to closeout. It offers features such as electronic data capture, study management, patient recruitment, and safety monitoring. Advarra's platform is particularly popular among researchers who require a high level of customization and who are conducting large, complex clinical trials.
In contrast, Clinplus is a more focused platform that offers a range of tools for managing clinical trials, including study management, site management, and patient tracking. One of the significant advantages of Clinplus is its ease of use - the platform is intuitive and user-friendly, making it an excellent choice for researchers who are new to clinical trial management.
When comparing Advarra vs Clinplus, it's important to consider your specific needs as a researcher. Advarra is a more comprehensive platform and offers more advanced features for managing large, complex trials. However, it can be more challenging to navigate for users who are new to clinical trial management. In contrast, Clinplus is more user-friendly but may not be as well-suited for large, complex trials.
Pricing is another important consideration when choosing between Advarra vs Clinplus. Both platforms offer subscription-based models, and pricing can vary depending on the specific features and modules you require. Advarra's pricing is typically higher than Clinplus, reflecting its more advanced features and greater level of customization.
Overall, both Advarra and Clinplus are powerful and reliable clinical trial management platforms. The choice between the two ultimately depends on your specific needs and the nature of your clinical trial. If you require a comprehensive platform with advanced features and a high level of customization, Advarra may be the best choice. However, if you are new to clinical trial management and need a more user-friendly platform, Clinplus may be the better option.
8. MasterControl Clinical Excellence vs Advarra
When it comes to running clinical trials, two of the most popular solutions on the market are MasterControl Clinical Excellence and Advarra. MasterControl is an all-in-one platform that helps clinical researchers streamline their processes, from study design to final submission. The platform includes features such as electronic data capture (EDC), real-time monitoring, and remote study visits. One of the most significant advantages of MasterControl is its flexibility - it can be used for a wide variety of clinical trials, including multi-site trials.
On the other hand, Advarra is a comprehensive platform that offers a range of tools to support clinical research, from study startup to closeout. The platform includes features such as electronic consent forms, study management tools, and real-time data analysis. One of the most significant advantages of Advarra is its ability to provide end-to-end support for clinical research, which can be particularly beneficial for complex trials.
When comparing MasterControl Clinical Excellence vs Advarra, it's essential to consider the specific needs of your clinical trial. MasterControl is an excellent option for researchers who need a flexible, all-in-one platform that can be used for a wide range of clinical trials. In contrast, Advarra is better suited for researchers who need a more comprehensive suite of tools to support their trial from start to finish. Ultimately, the choice between MasterControl Clinical Excellence vs Advarra will depend on the specific requirements of your trial.
In terms of pricing, both MasterControl and Advarra offer subscription-based models, with pricing varying depending on the specific features and modules you need. MasterControl's pricing model is based on the number of subjects and forms used in a trial, while Advarra's pricing is based on the number of users and studies. Both platforms offer custom pricing for enterprise-level clients. When choosing between MasterControl Clinical Excellence vs Advarra, it's essential to carefully consider your budget and the features you require.
In conclusion, both MasterControl Clinical Excellence and Advarra are powerful platforms that can help researchers streamline their clinical trial management processes. The choice between MasterControl Clinical Excellence vs Advarra will depend on your specific needs, budget, and the type of trial you are conducting. With the right platform in place, clinical researchers can focus on what really matters - advancing medical knowledge and improving patient outcomes.
9. Rave CTMS vs. Advarra
Advarra and Rave CTMS are both popular tools used by researchers to run clinical trials. Advarra is a comprehensive platform that offers regulatory and compliance solutions, while Rave CTMS provides a suite of tools for clinical trial management.
One of the advantages of Advarra is its focus on regulatory compliance. The platform provides a wide range of regulatory solutions, including IRB management, protocol development, and safety reporting. Advarra also offers customizable workflows and templates to help researchers streamline their processes and ensure they are in compliance with regulatory requirements. Additionally, Advarra has a team of experts available to provide support and guidance on regulatory compliance issues.
Rave CTMS, on the other hand, is designed specifically for clinical trial management. The platform provides tools for study planning, patient enrollment, site management, and data management. One of the significant advantages of Rave CTMS is its ability to integrate with other systems, such as EDC platforms, to provide a comprehensive view of trial data. Rave CTMS also offers customizable reporting features, which allow researchers to generate real-time reports and track study progress.
When comparing Advarra vs Rave CTMS, it's important to note that each platform has its strengths and weaknesses. Advarra is an excellent choice for researchers who need robust regulatory and compliance solutions, while Rave CTMS is better suited for those who need a comprehensive suite of tools for clinical trial management. Ultimately, the choice between Advarra and Rave CTMS will depend on the specific needs of the researcher and the trial they are conducting.
In terms of pricing, both Advarra and Rave CTMS offer flexible subscription-based models, and pricing can vary depending on the features and modules required. Advarra's pricing is based on the number of studies and modules used, while Rave CTMS pricing is based on the number of sites and users. Both platforms also offer custom pricing for enterprise-level clients. When it comes to customer support, Advarra and Rave CTMS both have a solid reputation for providing excellent support to their clients. Advarra offers 24/7 support with a dedicated team available via phone, email, or chat. Rave CTMS also provides 24/7 support, with a team of experts available to help researchers with onboarding, training, and ongoing support.
10. NGINX vs. Advarra
NGINX and Advarra are two different tools that can be used to run clinical trials. NGINX is a web server that can be used to manage and serve web content, while Advarra is a clinical research organization that provides a range of services to support clinical trials.
One of the primary advantages of NGINX is its scalability. It can handle high traffic loads and can be used to serve content quickly and efficiently. This can be particularly useful in clinical trials where there may be a large number of participants accessing study materials and data. Additionally, NGINX is open source and has a large community of users and developers, which means that it is constantly evolving and improving.
On the other hand, Advarra provides a comprehensive suite of services that can support clinical trials from start to finish. This includes everything from study design and protocol development to regulatory compliance and data management. Advarra's services are designed to help researchers navigate the complex landscape of clinical research and ensure that their trials are conducted ethically and efficiently.
However, one of the potential drawbacks of using Advarra is the cost. Clinical research is an expensive endeavor, and Advarra's services can add a significant expense to a study's budget. Additionally, using a third-party organization like Advarra can add a layer of complexity to the trial management process, which may not be suitable for all researchers.
In summary, both NGINX and Advarra have their advantages and disadvantages when it comes to running clinical trials. NGINX is a scalable and efficient web server that can handle high traffic loads, while Advarra provides a comprehensive suite of services to support all aspects of clinical trial management. Ultimately, the choice between these two tools will depend on the specific needs of the research project and the resources available to the research team.
11. ProPharma Group Co. vs. Advarra
ProPharma Group Co. and Advarra are two leading organizations that offer clinical trial services to pharmaceutical and biotech companies. ProPharma Group Co. provides services that encompass clinical development, regulatory affairs, and pharmacovigilance, whereas Advarra offers institutional review board (IRB) services, human subject protection, and compliance solutions.
One of the advantages of ProPharma Group Co. is its expertise in clinical development, which allows the company to provide end-to-end services that cover the entire clinical trial process. This includes protocol design, site identification, and selection, as well as monitoring and management of clinical trials. ProPharma Group Co. also has a deep understanding of the regulatory landscape and can help its clients navigate complex regulatory requirements in various regions worldwide.
In comparison, Advarra's primary focus is on providing IRB services and human subject protection solutions, making it an excellent choice for companies looking for an experienced partner to manage ethical considerations in clinical trials. Advarra has a team of experts with extensive experience in clinical research, which ensures that all studies conducted under their oversight meet the highest ethical standards. Advarra also offers an integrated technology platform for managing IRB submissions, reviews, and approvals, which can help streamline the regulatory process.
However, one of the disadvantages of ProPharma Group Co. is that it may not offer the same level of expertise in human subject protection as Advarra, as it is not a dedicated IRB service provider. Similarly, Advarra may not offer the same level of expertise in clinical development as ProPharma Group Co., which could be a disadvantage for companies looking for end-to-end clinical trial services.
Ultimately, the choice between ProPharma Group Co. vs Advarra will depend on the specific needs and goals of the company conducting the clinical trial. While both organizations offer valuable services, their areas of expertise differ, and it's essential to consider these differences carefully before making a decision.
12. ACA Group vs. Advarra
When it comes to running clinical trials, ACA Group and Advarra are two options that researchers should consider. ACA Group is a full-service CRO that specializes in regulatory affairs, clinical trial management, and quality assurance. It has a strong reputation for providing personalized attention to its clients and has a global reach. Advarra, on the other hand, is a provider of institutional review board (IRB) services and technology solutions for clinical research. It has a broad range of solutions for researchers, including an electronic IRB platform, consulting services, and training programs.
When comparing ACA Group vs Advarra, one of the most significant advantages of ACA Group is its full-service approach to clinical trial management. It offers a wide range of services that can help researchers from study startup to closeout, including regulatory affairs, clinical trial management, and quality assurance. ACA Group is known for its personalized attention to clients, which can be a valuable asset to researchers who need hands-on support throughout the clinical trial process.
However, Advarra is also a compelling option for researchers, especially those who need IRB services. Advarra's electronic IRB platform, IRBNet, is a comprehensive solution that can help researchers manage their IRB submissions, review, and approval process. It also provides consulting services and training programs that can help researchers navigate the complex regulatory landscape of clinical research.
In summary, the choice between ACA Group vs Advarra ultimately depends on the specific needs of the researcher and the trial they are conducting. ACA Group is a full-service CRO that can provide hands-on support throughout the clinical trial process, while Advarra is a provider of IRB services and technology solutions that can help researchers manage their regulatory requirements. Both companies have their strengths and weaknesses, so it's important to carefully consider your options before making a decision.
13. WCG Clinical vs Advarra
WCG Clinical and Advarra are two of the most prominent players in the clinical trials industry, providing a wide range of services to researchers and organizations worldwide. WCG Clinical's suite of solutions covers everything from patient recruitment to data management, with a particular emphasis on regulatory compliance and risk mitigation. One of the biggest advantages of WCG Clinical is its deep expertise in the regulatory environment, which allows it to guide researchers through the complex process of clinical trial design and execution.
On the other hand, Advarra is a leading provider of institutional review board (IRB) services, providing ethical and regulatory oversight for clinical trials. Advarra's solutions cover everything from IRB reviews to compliance monitoring and training, with a focus on delivering high-quality, efficient services to clients. One of the biggest advantages of Advarra is its commitment to customer service, with a team of dedicated experts available to support clients throughout the entire clinical trial process.
When comparing WCG Clinical vs Advarra, it's clear that both companies have a lot to offer. WCG Clinical has a broader range of services, while Advarra has a more specialized focus on IRB services. However, the choice between the two ultimately comes down to the specific needs of the researcher or organization. If you require a more comprehensive suite of services, including patient recruitment and data management, WCG Clinical may be the better option. On the other hand, if you're primarily focused on ethical and regulatory compliance, Advarra may be the more suitable choice.
In terms of pricing, both WCG Clinical and Advarra offer competitive pricing models, with rates varying depending on the specific services required. WCG Clinical offers flexible pricing options that allow researchers to pay only for the services they need, while Advarra offers a comprehensive pricing model that covers all aspects of IRB services. Both companies are committed to delivering value to their clients, and pricing is always a key consideration when choosing between them.
In conclusion, both WCG Clinical and Advarra are leading players in the clinical trials industry, offering a wide range of services to researchers and organizations worldwide. Choosing between them ultimately depends on the specific needs of the researcher or organization, with WCG Clinical offering a more comprehensive suite of services and Advarra specializing in ethical and regulatory compliance. Regardless of the choice, both companies are committed to delivering value to their clients and are well-positioned to support the clinical trial process.
14. Highstreet IT Solutions LLC vs. Advarra
When it comes to running clinical trials, Highstreet IT solutions LLC and Advarra are two companies that offer different advantages and disadvantages.
Highstreet IT solutions LLC provides IT solutions that can help streamline clinical trials. Their strengths include their ability to customize their solutions to meet the specific needs of each individual trial, and their commitment to staying up-to-date with the latest industry standards and regulations. One of the drawbacks of Highstreet IT solutions LLC is that they may not offer as comprehensive a suite of tools as some other companies, which could limit their usefulness for larger, more complex trials.
Advarra, on the other hand, is a leading provider of institutional review board (IRB) services and regulatory consulting. Their strengths lie in their expertise in navigating the complex regulatory environment surrounding clinical trials, as well as their ability to provide guidance and support to researchers throughout the trial process. One potential drawback of Advarra is that their services may be more expensive than some other companies, which could make them less accessible to smaller research organizations.
Ultimately, the choice between Highstreet IT solutions LLC and Advarra will depend on the specific needs and budget of the researcher or research organization. For those who prioritize flexibility and customization, Highstreet IT solutions LLC may be the better option. For those who need expert guidance and support navigating the regulatory landscape, Advarra may be the better choice.
15. ExamWorks LLC vs. Advarra
ExamWorks LLC and Advarra are two companies that offer services for clinical trials, but they have different strengths and weaknesses.
ExamWorks LLC is a global provider of medical-legal services, including independent medical examinations, medical record reviews, and clinical trial services. The company has a strong reputation for its attention to detail and quality control, which is critical in the clinical trial industry. ExamWorks LLC's clinical trial services offer a comprehensive suite of tools and solutions for managing clinical trials, including regulatory consulting, site selection, and patient recruitment.
Advarra, on the other hand, is a technology-enabled clinical research organization that provides a range of services for clinical trials. The company offers innovative solutions for clinical trial management, such as risk-based monitoring, safety reporting, and eConsent. Advarra's technology platform is highly advanced and offers a comprehensive suite of tools for managing every aspect of a clinical trial.
When comparing ExamWorks LLC vs Advarra, it is important to consider the specific needs of your clinical trial. ExamWorks LLC is an excellent choice for researchers who need high-quality clinical trial services and support, while Advarra is a better option for researchers who are interested in advanced technology solutions for clinical trial management. Both companies have a reputation for delivering excellent service, but the choice will depend on the specific requirements of your clinical trial.
In terms of pricing, both ExamWorks LLC and Advarra offer custom pricing based on the specific needs of the client. The cost of services can vary depending on the size and complexity of the clinical trial, as well as the specific services required. It is essential to get an accurate estimate from both companies to make an informed decision about which service provider to choose.
In summary, ExamWorks LLC and Advarra are both excellent service providers for clinical trials, but they offer different strengths and weaknesses. Researchers should carefully consider their specific needs and the services offered by each company before making a decision. Ultimately, choosing the right service provider is critical for the success of your clinical trial, and it is worth taking the time to make an informed decision.
16. Consilio LLC vs. Advarra
Consilio LLC and Advarra are two of the leading companies that specialize in running clinical trials. Consilio LLC offers a range of services, including e-discovery, document review, and regulatory compliance, while Advarra focuses on providing institutional review board (IRB) services to research organizations.
One of the advantages of Consilio LLC is its extensive experience in the e-discovery and legal technology fields. This expertise allows Consilio LLC to handle complex data management tasks that may be necessary in clinical trial management. Additionally, Consilio LLC has a global presence, which means that it can support clinical trials conducted in multiple countries and regions.
On the other hand, Advarra has a strong reputation for providing high-quality IRB services. This includes providing ethical review and oversight of clinical trials to ensure that they are conducted in accordance with federal regulations and ethical principles. Advarra's IRB services can be especially valuable for smaller research organizations that may not have the resources to manage IRB requirements in-house.
When comparing Consilio LLC vs Advarra, the choice ultimately depends on the specific needs of the research organization. Consilio LLC may be a better choice for organizations that require extensive data management and regulatory compliance services, while Advarra may be a better fit for those that need IRB services. However, it's important to note that both companies offer a range of valuable services that can help streamline the clinical trial process and ensure compliance with regulations.
In terms of pricing, both Consilio LLC and Advarra offer custom pricing based on the specific needs of the research organization. This can make it challenging to compare the two companies based solely on price. However, it's worth noting that Consilio LLC's expertise in data management and legal technology may come with a higher price tag, while Advarra's focus on IRB services may make it more affordable for smaller research organizations. Overall, both companies offer valuable services that can help ensure the success of clinical trials.
17. Imprivata Confirm ID vs. Advarra
Imprivata Confirm ID and Advarra are two popular solutions for running clinical trials. Imprivata Confirm ID is a comprehensive identity and access management platform that provides secure access to patient data, reduces risk, and streamlines workflows. One of the significant advantages of Imprivata Confirm ID is its user-friendly interface and ease of use, making it an excellent option for clinical research teams who prioritize simplicity and efficiency. Moreover, Imprivata Confirm ID offers multifactor authentication, biometric identification, and role-based access control, making it a reliable platform for managing sensitive patient data.
On the other hand, Advarra is a technology-enabled clinical research organization (CRO) that offers end-to-end clinical trial solutions. Advarra's platform includes everything from regulatory consulting and site selection to study execution and data management. One of the significant advantages of Advarra is its experience in managing clinical trials across multiple therapeutic areas, making it an excellent option for researchers conducting complex studies. Additionally, Advarra offers a range of specialized services, including patient recruitment, clinical monitoring, and data analytics, making it a one-stop-shop for clinical trial management.
When comparing Imprivata Confirm ID vs Advarra, it should be remembered that these are two technical solutions with different strengths and weaknesses. Imprivata Confirm ID is an excellent option for researchers who need a user-friendly platform that provides secure access to patient data. In contrast, Advarra is a better choice for researchers who need a comprehensive suite of services, from study design to data analysis. The winner between Imprivata Confirm ID vs Advarra will depend on the researcher's specific needs and the trial they are conducting.
Both Imprivata Confirm ID and Advarra offer a range of valuable features and can be an asset to clinical trial management. When it comes to pricing, Imprivata Confirm ID offers a subscription-based model, and pricing can vary depending on the number of users and features used. Advarra's pricing is typically customized based on the specific needs of each trial. In terms of customer support, both platforms offer excellent support to their clients, with dedicated support teams available to help with onboarding, training, and ongoing support. Ultimately, the decision between Imprivata Confirm ID vs Advarra will depend on the researcher's specific requirements and the clinical trial's unique needs.
18. ACTO Omnichannel Education for LifeSciences vs. Advarra
ACTO Omnichannel Education for LifeSciences and Advarra are two popular solutions in the clinical trial space. ACTO is an omnichannel education and engagement platform that enables life sciences companies to train and educate their sales teams and healthcare providers. One of the significant advantages of ACTO is its ability to deliver personalized content across multiple channels, including mobile, web, and email. This makes it easier for life sciences companies to engage with their target audience and improve their sales performance.
On the other hand, Advarra is a comprehensive clinical research organization that provides services such as regulatory consulting, clinical trial management, and institutional review board (IRB) services. Advarra is known for its expertise in navigating the complex regulatory environment surrounding clinical trials and its ability to provide customized solutions to meet the unique needs of each study.
When comparing ACTO Omnichannel Education for LifeSciences vs Advarra, it's important to consider the specific needs of your clinical trial. If your primary focus is on training and educating your sales team or healthcare providers, then ACTO may be the right choice for you. However, if you need comprehensive clinical trial management services, including regulatory consulting and IRB services, then Advarra may be a better fit.
Another important factor to consider when comparing ACTO vs Advarra is pricing. ACTO's pricing model is based on the number of users and the features required, while Advarra's pricing varies based on the services required. It's important to carefully evaluate the pricing structure of each platform and determine which one best fits your budget and needs.
In conclusion, both ACTO Omnichannel Education for LifeSciences and Advarra offer valuable services to the clinical trial industry. The choice between the two ultimately depends on the specific needs of your study, whether it's education and engagement or comprehensive clinical trial management services. By carefully evaluating your needs and the strengths of each platform, you can make an informed decision that best serves your research goals.
19. OpenSim vs. Advarra
OpenSim and Advarra are both clinical trial management platforms that offer unique benefits for researchers. OpenSim is an open-source software platform that allows researchers to create biomechanical models to simulate human movement and analyze data. One of the key advantages of OpenSim is its ability to integrate with other software tools, allowing researchers to customize their workflows and automate data processing. However, OpenSim has a steeper learning curve than some other clinical trial management platforms, making it more suitable for researchers with technical expertise.
Advarra, on the other hand, is a comprehensive platform for clinical trial management that offers a range of features, including protocol development, site activation, and safety reporting. Advarra is particularly known for its regulatory expertise, providing researchers with guidance and support to ensure compliance with applicable regulations and guidelines. One of the key advantages of Advarra is its ability to streamline the clinical trial process and reduce the administrative burden on researchers.
When comparing OpenSim vs Advarra, it's important to consider the specific needs of your clinical trial. If you require biomechanical modeling and analysis capabilities, OpenSim may be the better choice. However, if you need a comprehensive platform that can manage all aspects of your clinical trial, including regulatory compliance, Advarra may be a better fit.
Ultimately, the choice between OpenSim vs Advarra will depend on the researcher's technical expertise and the specific requirements of the clinical trial. Both platforms offer valuable features and benefits that can help researchers streamline their workflows and run more efficient trials. As with any clinical trial management platform, it's essential to carefully consider your needs and conduct thorough research before making a decision.
20. OBIX Perinatal Data System vs. Advarra
OBIX Perinatal Data System and Advarra are two popular options for running clinical trials, each with their own advantages and disadvantages. OBIX is a specialized system designed specifically for perinatal data management, which makes it an excellent choice for researchers conducting trials related to pregnancy and childbirth. OBIX offers a range of features, including data collection, analysis, and reporting, as well as customizable workflows and secure data storage.
Advarra, on the other hand, is a more general platform that offers a wide range of clinical trial management tools, including study startup, regulatory compliance, and safety reporting. Advarra is designed to be flexible and customizable, making it a good choice for researchers working on a variety of different trials. One of the most significant advantages of Advarra is its integrated platform, which allows users to manage all aspects of their trials from a single interface.
When comparing OBIX vs Advarra, the choice will depend largely on the specific needs of the trial. OBIX is an excellent option for researchers working in the field of perinatal data management, while Advarra is better suited to researchers who need a more comprehensive platform for managing a wide range of clinical trials. One of the biggest disadvantages of OBIX is that it is a specialized system, which means it may not be as flexible as other platforms. On the other hand, Advarra's integrated platform can be a double-edged sword - while it's convenient to have everything in one place, it can also be overwhelming for users who only need to use certain features.
In terms of pricing, both OBIX and Advarra offer custom pricing based on the needs of the client. OBIX's pricing is based on the number of beds and labor and delivery rooms, while Advarra's pricing is based on the specific modules and features needed. As with any clinical trial management platform, it's important to carefully consider your needs before making a decision. While OBIX vs Advarra may seem like an apples-to-apples comparison, the truth is that each platform has its own unique strengths and weaknesses. Ultimately, the choice will depend on the specific needs of the trial and the preferences of the researchers involved.
21. QIAGEN Digital Insights vs. Advarra
When it comes to running clinical trials, two popular solutions on the market are QIAGEN Digital Insights and Advarra. QIAGEN Digital Insights offers a suite of bioinformatics solutions designed to help researchers manage and analyze their genomic data, while Advarra provides a range of clinical research services to help organizations navigate the complex regulatory landscape.
One of the biggest advantages of QIAGEN Digital Insights is its ability to process and analyze large volumes of genomic data quickly and efficiently. The platform offers a range of tools for variant analysis, interpretation, and reporting, which can help researchers identify clinically relevant mutations and make informed decisions about patient care. Additionally, QIAGEN Digital Insights provides a secure and compliant environment for data storage and sharing, which can be particularly important for organizations working with sensitive patient data.
On the other hand, Advarra has a reputation for providing high-quality regulatory support services to organizations conducting clinical trials. The company offers a range of solutions to help researchers navigate the regulatory landscape, including institutional review board (IRB) services, ethics consulting, and compliance training. Advarra also provides a centralized platform for managing all aspects of the regulatory process, which can help organizations save time and reduce administrative burden.
When comparing QIAGEN Digital Insights vs Advarra, it's important to consider the specific needs of your organization and the clinical trials you are conducting. QIAGEN Digital Insights is a great option for organizations that need to manage and analyze large volumes of genomic data quickly and efficiently. However, if regulatory compliance is a top priority, Advarra may be the better choice, as the company has a strong reputation for providing high-quality regulatory support services to organizations conducting clinical trials.
In conclusion, both QIAGEN Digital Insights and Advarra offer valuable solutions for organizations conducting clinical trials. Choosing between the two will ultimately depend on the specific needs of your organization and the trials you are conducting. Organizations that prioritize genomic data analysis may benefit more from QIAGEN Digital Insights, while those that prioritize regulatory compliance may benefit more from Advarra's suite of regulatory support services.
22. MedPut vs. Advarra
Advarra and Medput are two platforms designed to streamline the clinical trial process. Advarra is an end-to-end solution that offers a suite of tools for managing every aspect of clinical research, from protocol development to study closeout. One of Advarra's most significant advantages is its expertise in regulatory compliance, which can be especially valuable for studies that require a high level of oversight.
Medput is another platform designed to make clinical research more efficient. Medput's focus is on simplifying the billing and reimbursement process, which can be a major headache for many researchers. With Medput, users can automate invoicing, track payments, and manage study budgets in real time. By eliminating manual processes and streamlining workflows, Medput can help researchers save time and reduce errors.
When comparing Advarra vs Medput, it's important to consider the specific needs of your study. If you require comprehensive support for every aspect of clinical research, Advarra may be the better choice. On the other hand, if you are specifically looking for a platform to manage billing and reimbursements, Medput may be the better option. Both platforms offer valuable tools and can be an asset to clinical trial management, but it's important to carefully consider your needs before making a decision.
In terms of pricing and customer support, both Advarra and Medput offer subscription-based models, with pricing varying depending on the specific features and modules you need. Advarra's pricing is based on the size and complexity of your study, while Medput's pricing is based on the number of invoices processed. Both platforms offer custom pricing for enterprise-level clients. In terms of customer support, Advarra has a reputation for providing excellent support to its clients, with a dedicated customer success team available to help with onboarding, training, and ongoing support. Medput also provides strong support to its clients, with a dedicated support team available via phone, email, or chat.
In conclusion, both Advarra and Medput offer valuable tools for managing clinical research. Advarra is a comprehensive solution that can handle every aspect of clinical research, while Medput is focused specifically on billing and reimbursement. Choosing between the two will depend on the specific needs of your study, and it's important to carefully consider your options before making a decision. Regardless of which platform you choose, both Advarra and Medput have reputations for providing excellent support and can be an asset to clinical trial management.
23. ActivityPro vs. Advarra
Advarra and ActivityPro are two clinical trial management platforms that offer unique features and capabilities. Advarra is a comprehensive platform that offers solutions for all phases of clinical research, including regulatory compliance, patient recruitment, and data management. One of the most significant advantages of Advarra is its ability to provide personalized service to its clients, allowing them to tailor their clinical trial management experience to their specific needs.
ActivityPro, on the other hand, is a platform that specializes in data management and analysis. It offers a range of features, including electronic data capture, study monitoring, and real-time data analysis. One of the most significant advantages of ActivityPro is its user-friendly interface, which makes it easy for even non-technical users to navigate.
Comparing ActivityPro vs Advarra, one can see that they are two different tools with different strengths. Advarra is ideal for researchers who need a comprehensive suite of solutions to manage their clinical trials, while ActivityPro is more suited to researchers who need a robust platform for data management and analysis. Choosing between ActivityPro vs Advarra will ultimately depend on the researcher's specific needs and the clinical trial they are conducting.
In terms of pricing, both platforms offer custom pricing for enterprise-level clients, making it difficult to estimate pricing without a specific list of requirements. However, it's worth noting that Advarra's pricing model is based on a subscription-based model, while ActivityPro's pricing is based on the number of study subjects and forms used in a trial. Both platforms offer excellent customer support, with dedicated support teams available to help clients with onboarding, training, and ongoing support.
In conclusion, Advarra and ActivityPro are two powerful clinical trial management platforms that offer unique features and capabilities. Choosing between ActivityPro vs Advarra will depend on the researcher's specific needs and the clinical trial they are conducting. Whether it's a comprehensive suite of solutions or robust data management and analysis tools, both platforms can help researchers streamline their workflows and run more efficient trials.
24. MedSupply Software vs. Advarra
When considering clinical trial management software, two popular solutions are MedSupply Software and Advarra. MedSupply Software offers a cloud-based platform that supports all aspects of clinical trials, including patient recruitment, data management, and compliance reporting. One of the most significant advantages of MedSupply Software is its intuitive user interface, which allows users to easily manage and track study data in real-time.
On the other hand, Advarra is a comprehensive clinical research organization that provides end-to-end services to support clinical trials. The organization offers a suite of solutions, including regulatory compliance, site support, and study start-up. Advarra is particularly popular among researchers who need a full-service provider to manage all aspects of their clinical trials, from study design to regulatory compliance.
When comparing MedSupply Software vs Advarra, it's important to consider the specific needs of your clinical trial. MedSupply Software is an excellent choice for researchers who are primarily interested in a user-friendly platform that can manage all aspects of their study. On the other hand, Advarra is a better choice for researchers who need a full-service provider that can manage all aspects of their clinical trial, from regulatory compliance to site support.
One potential disadvantage of MedSupply Software is its pricing structure, which is based on the number of users and modules used. This can make it more expensive for larger clinical trials that require more users and modules. Advarra, on the other hand, offers custom pricing based on the specific needs of the trial, which can be more cost-effective for larger studies.
In conclusion, both MedSupply Software and Advarra are powerful solutions for managing clinical trials, but they offer different strengths depending on the needs of the trial. MedSupply Software is a great option for researchers who need a user-friendly platform that can manage all aspects of their study, while Advarra is a better choice for those who need a full-service provider to manage all aspects of their clinical trial. Ultimately, the decision between the two will depend on the specific needs of the trial and the resources available to manage it.
25. MedBridge Microlearning vs. Advarra
When it comes to running clinical trials, there are a lot of different tools and technologies available to help researchers manage the process. Two of the most popular options on the market today are MedBridge Microlearning and Advarra.
MedBridge Microlearning is a web-based platform designed to help clinical trial teams learn and retain critical information quickly and efficiently. The platform uses microlearning techniques to break down complex concepts into bite-sized chunks, making it easier for users to absorb and remember important information. One of the most significant advantages of MedBridge Microlearning is its flexibility - it can be used for a wide variety of clinical trials and can be customized to meet the specific needs of each research team.
Advarra, on the other hand, is an end-to-end solution for clinical trial management. The platform provides tools for study design, protocol development, and regulatory compliance, as well as patient engagement and recruitment. Advarra's platform is known for its ability to simplify complex processes and reduce manual workflows, helping researchers run more efficient trials.
When comparing MedBridge Microlearning vs Advarra, it's essential to consider the specific needs of your clinical trial. MedBridge Microlearning is a great option for teams looking to improve their training and knowledge retention, while Advarra is better suited for those who need a more comprehensive platform that can handle everything from study design to patient engagement. Both platforms have their strengths and can be valuable assets to clinical trial management.
In terms of pricing, both MedBridge Microlearning and Advarra offer subscription-based models, with pricing that can vary depending on the specific features and modules you need. MedBridge Microlearning's pricing is based on the number of users and courses, while Advarra's pricing is based on the size and complexity of your study. It's important to carefully consider your budget and requirements before deciding which platform to use. Additionally, both platforms offer customer support to their clients, with dedicated teams available to assist with onboarding, training, and ongoing support.
In conclusion, choosing between MedBridge Microlearning vs Advarra ultimately depends on the specific needs of your clinical trial. MedBridge Microlearning is an excellent option for teams looking to improve their training and knowledge retention, while Advarra is better suited for those who need a comprehensive platform that can handle all aspects of their trial management. By carefully considering your requirements and budget, you can choose the platform that is right for you and your research team.
26. EY vs. Advarra
When it comes to running clinical trials, two of the most popular solutions on the market are EY and Advarra. EY is a global professional services firm that provides a wide range of services, including consulting, audit, tax, and transaction advisory services. They also offer a suite of services specifically designed for clinical trials, including study design, clinical data management, and regulatory compliance. One of the most significant advantages of EY is their extensive global network, which allows them to provide support and expertise to clients in a wide range of geographic locations.
Advarra, on the other hand, is a provider of institutional review board (IRB) and research compliance services. They specialize in helping researchers navigate the complex regulatory landscape of clinical trials, ensuring that all research is conducted ethically and in compliance with relevant laws and regulations. One of the most significant advantages of Advarra is their expertise in IRB services, which is critical for ensuring the safety and welfare of study participants.
When comparing EY vs Advarra for running clinical trials, it's important to consider the specific needs of the study. For example, EY might be a better choice for researchers who need support with study design and clinical data management, while Advarra is a better choice for those who need help with regulatory compliance and IRB services. Ultimately, the choice between EY and Advarra will depend on the specific needs of the researcher and the study they are conducting.
One potential disadvantage of EY is that they offer a broad range of services, which means that their focus on clinical trials may not be as deep as some other providers. On the other hand, one potential disadvantage of Advarra is that their focus on IRB services may not provide as much support for other aspects of clinical trial management, such as study design and data management. However, both EY and Advarra have strong reputations in the industry and are trusted by many researchers and institutions to provide high-quality services.
27. Deloitte vs. Advarra
When it comes to running clinical trials, Deloitte and Advarra are two names that often come up in discussions. Deloitte is a multinational professional services network that provides consulting, audit, tax, and advisory services to a wide range of clients. Advarra, on the other hand, is a clinical research organization (CRO) that specializes in providing ethical and regulatory compliance support to the biopharmaceutical and medical device industries.
One of the biggest advantages of Deloitte is its extensive experience and expertise in managing complex projects. With a vast network of professionals across various fields, Deloitte can bring a diverse range of skills and perspectives to the table, helping to ensure that clinical trials are well-designed, efficiently managed, and comply with all relevant regulations. Additionally, Deloitte has invested heavily in technology and data analytics, which can help to streamline the clinical trial process and provide valuable insights into patient outcomes and trial results.
In contrast, Advarra's strength lies in its specialized expertise in regulatory compliance and ethics. Advarra has a team of experienced regulatory and ethical experts who can help to ensure that clinical trials are conducted in a safe, ethical, and compliant manner. This can be particularly valuable for researchers who are new to the field or who are working on particularly complex or sensitive trials. Advarra also offers a range of other services, such as site selection, patient recruitment, and clinical trial management, which can help to simplify the clinical trial process and ensure that everything runs smoothly.
However, both Deloitte and Advarra have their drawbacks. Deloitte's size and breadth can sometimes lead to bureaucracy and slow decision-making, which can be frustrating for researchers who need to move quickly. Advarra, on the other hand, may not be the best fit for researchers who need a more comprehensive suite of services, such as data analytics or patient engagement. Ultimately, the choice between Deloitte and Advarra will depend on the specific needs and goals of the individual clinical trial.
28. Medidata vs Advarra
When it comes to clinical trial management systems, Advarra competitors include well-known platforms like Medidata. Medidata offers a comprehensive suite of tools for clinical trial management, including electronic data capture (EDC), trial design, and data management. One of the most significant advantages of Medidata is its ability to handle large, complex trials with multiple sites and study arms.
However, when comparing Advarra vs Medidata, it's essential to consider the unique needs of each trial. Advarra's Clinical Conductor CTMS is a cloud-based platform that provides tools for study management, subject recruitment, and financial management. One of the most significant advantages of Advarra's platform is its ability to integrate with other systems, including EDC platforms and electronic health record (EHR) systems. This integration makes it easy for researchers to manage all aspects of their trial in one place.
While Medidata may be better suited for larger, more complex trials, Advarra's Clinical Conductor CTMS may be a better choice for smaller, more focused studies. Advarra's platform is designed to be user-friendly and intuitive, which can help researchers save time and reduce the risk of errors. Additionally, Advarra's platform is highly customizable, allowing researchers to tailor it to their specific needs.
In terms of pricing, both Advarra and Medidata offer subscription-based models, with pricing that can vary depending on the specific needs of the trial. Advarra's pricing is based on the number of studies and users, while Medidata's pricing is based on the number of studies, sites, and modules. Ultimately, the decision between Advarra vs Medidata will depend on the specific needs of the trial, and researchers should carefully consider their options before making a decision.
Conclusion
The utilization of Clinical Trial Management Software (CTMS) has become increasingly vital to clinical research. With the growing demand for efficient, secure and streamlined clinical trials, the CTMS market is expanding rapidly. The software provides a comprehensive set of tools for study planning, patient recruitment, monitoring, data collection, and analysis.
There are many options available in the market, from larger corporations like Oracle Health Sciences, to nimble start-ups like Science 37 and Clinerion. Each option offers unique and innovative solutions for clinical trial management, with varying features and pricing models to suit different needs.
One of the key benefits of utilizing a CTMS is the ability to centralize trial management, which enables greater collaboration and transparency among all stakeholders involved in the study. Additionally, the use of a CTMS can improve data accuracy, minimize errors and accelerate the speed of the study, ultimately resulting in cost savings and faster drug development.
Overall, the adoption of CTMS in clinical research is essential to achieve better study outcomes, and the CTMS market will continue to evolve as new technologies and approaches emerge to further enhance clinical trial management.
Mahalo Health is an innovative and customer-focused healthcare company that is poised to revolutionize the way we access medical care. Their commitment to personalized care and accessibility make them a standout player in the telemedicine industry.
We offer a variety of services, including virtual consultations with licensed healthcare providers, prescription refills, and at-home lab testing. Patients can access these services through Mahalo Health's easy-to-use app, which is available for both iOS and Android devices. Click here to know more.