Finally, an Electronic Data Capture (EDC) System designed for humans
A human-centric experience
EDC, eConsent, eCOA, eSource, and ePro tools don't run successful studies. Research like you do. You collect data from actual human participants who want the most effortless process to provide you with the clean data needed for your study. Mahalo's EDC system offers an intuitive experience for researchers and participants alike.
Imagine your research study, starting in days
Use our drag and drop survey builder to create your eCRFs in hours instead of weeks or months. Whether you're utilizing a fully decentralized or hybrid study design, configure each aspect of your study with Mahalo's user-friendly admin panel. We pride ourselves in being in the top 3% of EDCs, offering the shortest timeline from creation to deployment.
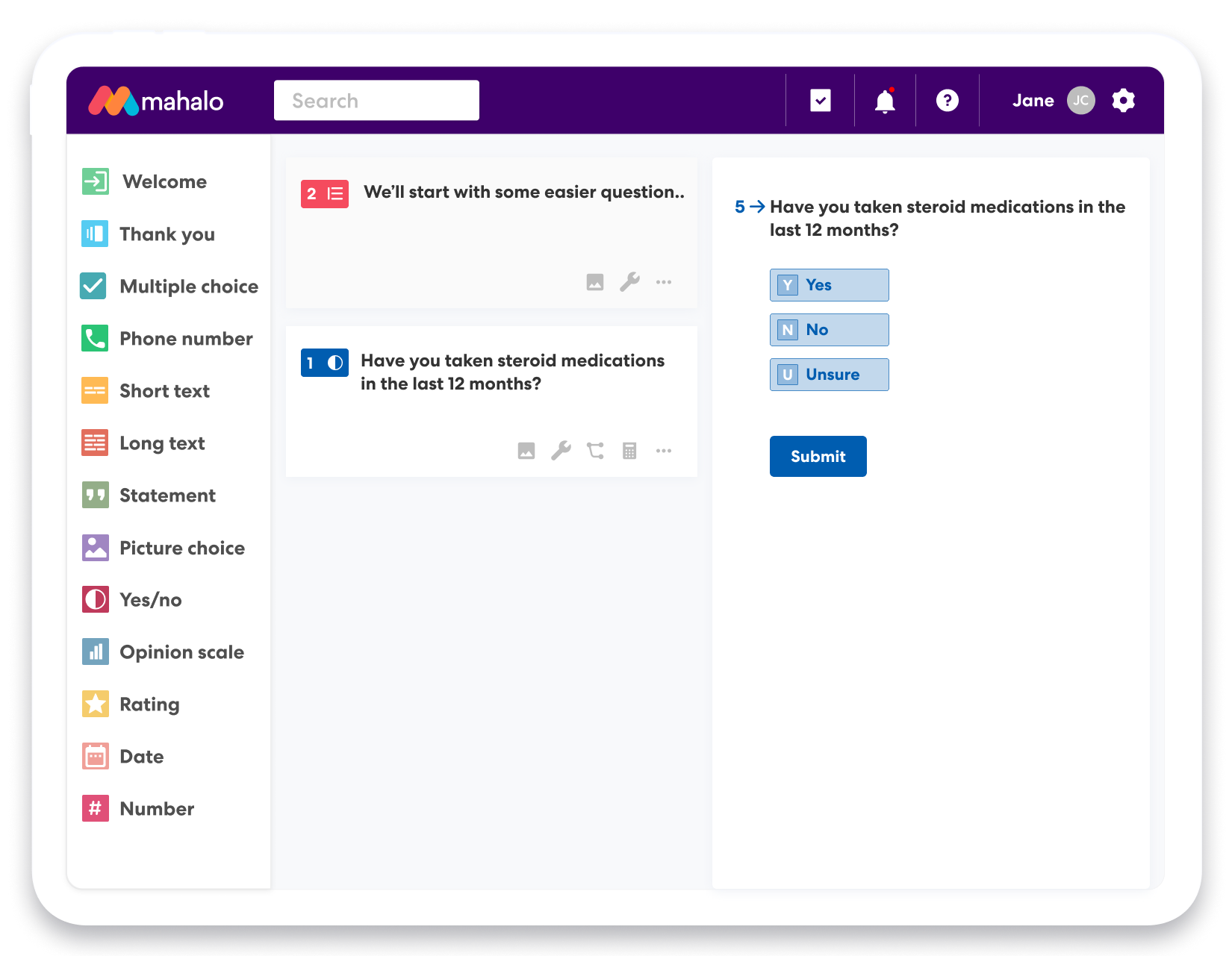
Complex surveys created in minutes
- 12 drag and drop form field options
- Start with a study template
- Set min and max limits for each form field
- Skip future questions based on the answer to a previous question
- Weight questions and calculate scores for completed surveys

Configurable user roles and permissions
- Setup individual access by use role
- Admin access
- Participants assigned to each researcher or clinician
- Configure when and where admins view data
- Set survey (eCRF) visibility by user role
- Custom user-rights
- Workflow rules for data collection
- Run all your studies with one account
All your study data in one place
Today's studies contain a complex patchwork of data sources that must be synced to a unified clinical database and mapped to their proper participants. With Mahalo, it's possible to complete patient surveys at clinics, pharmacies, or homes via self-reporting tools or Telehealth appointments. Additionally, IoT-connected medical devices, scales, and wearables collect additional data that must be synced, normalized, and linked to patient records. The Mahalo EDC platform supports all these data collection methods, and it allows researchers like you to pull in medical records and lab results from electronic health records (EHR) systems.
Unified clinical data platform
- Survey (eCRF) responses
- Pull data from EHR systems like EPIC and Cerner
- Patient and clinician-reported data
- Patient diary
- Chat history
- Unlimited data point collection per participant
- Permission-based data import and export (CSV or Excel)

.png)
Capture recurring measurements
- Lab test results
- Connected device readings
- Changes to medical history
- Unscheduled medical visits
- Adverse events
Monitor data quality and participant questions
- Real-time dashboard to track study progress
- Track data anomalies
- View individual participant progress
- Answer and manage participant questions
- Message participants via the in-app chat feature

Lock and secure data to improve quality
By validating data as participants and clinicians enter it in real-time with edit check rules that set minimum and maximum limits for values, you ensure simple human errors don't compromise your study data. Such validations are impossible to do in real-time with paper-based studies, but Mahalo offers intuitive tools to add edit checks to any field in just a few clicks.

Study data you can depend on
- Edit checks and validation rules
- Auto-lock data upon survey submission
- User permissions required to unlock data
- Audit trail for all changes made to captured data
- Authentication verifies subject enrollment in real-time
Randomize while preserving source data
- Weighted randomization
- Blinded randomization
- Automatic participant anonymizer


Securely store your data with confidence
- Data encryption in transit and at rest
- Two-factor authentication
- FDA 21 CFR Part 11 compliance
- HIPAA compliance
- Good Clinical Practice (GCP)
- Real-time study data backup
Compliance

HIPAA GDPR

21 C.F.R. Part 11

